Mercury Barometer Formula
This physics video tutorial provides a basic introduction into the mercury barometer. It explains how a basic barometer works.
Pressure Question Excel Physics
521 Mercury Barometer 1 Principle of mercury barometer When a one-meter long open ended glass tube is filled with mercury and is then turned upside down into a container filled with mercury part of the mercury flows out of the glass tube into the container.

Mercury barometer formula. Thus the sea level the vertical height of the water column would be 076 m 1361034 m. Atmospheric pressure can hold a vertical column of water about 136 times the height of the mercury column at a place. As we take the barometer to higher altitudes we find that the height of the mercury column decreases because less and less of the atmosphere is above the barometer.
A barometer is a simple instrument for measuring atmospheric pressure. The mass is Vrho A hrho the force is g times larger ie. The decrease in atmospheric pressure with height can be predicted from the barometric formula.
3Calibrate an aneroid barometer with a traveling standard barometer in the chamber for pressure. If the pressure is given in millimeters of mercury left textmmHg right the barometric formula is written in the form. A mercury barometer gives a pressure head of 758 mm.
The basic-school formula hrho g for the pressure may be derived as the force of the mercury column per unit area of the base. A h rho g and the force per unit area is therefore hrho g because A cancels. 760 mmHg 760 torr.
Which is given by the above-stated formula of atmospheric pressure and is proportional to the height of the mercury column. Calculate the atmospheric pressure in bar. The pressure on the mercury inside the tube pushes down and so does gravity but the outside pressure pushes the mercury back up until it is equal to the gravity and the pressure of.
EqP_hP_0efrac-mghkT eq A barometer measures in inches. Pleft h right 760exp left - 000012h right. The height of mercury barometer is 76 cm h_1 Density of mercury is 136 dfracgccrho _1 and rho _2 1.
If the reservoir is lowered sufficiently the level of the mercury in the reservoir is lower than in the closed limb and the pressure of the trapped air is Hh cm of mercury. H is read off from a barometer at the time of the experiment. Thus a glass tube more than 10 m long is required to make a water barometer.
This device is termed as the mercury barometer. Torricellian vacuum is then. One side is connected to the container and the.
The pressure at the bottom of the column of mercury is equal to the pressure of a column of air extending from the elevation of the barometer all the way to the top of the earths atmosphere. If your lucky the mercury barometer you bought came with a card indicating how much the instrument scale differed from the true pressure when it was originally calibrated by the manufacturerIn the case of my barometer the instrument reads 0005 inHg too low. The tube is strong enough because of its IMFs to withstand the pressure which is why it doesnt bend or crack.
What is a high barometric pressure. 1033 30 34433 displaystyle 10333034433. The mercury barometer is the standard instrument for atmospheric pressure measurement in weather reporting.
A barometer can be made by filling up a long glass tube with mercury then turning it upside down in a bath of mercury as shown. Now coming to the question we are given. It explains how to calculate.
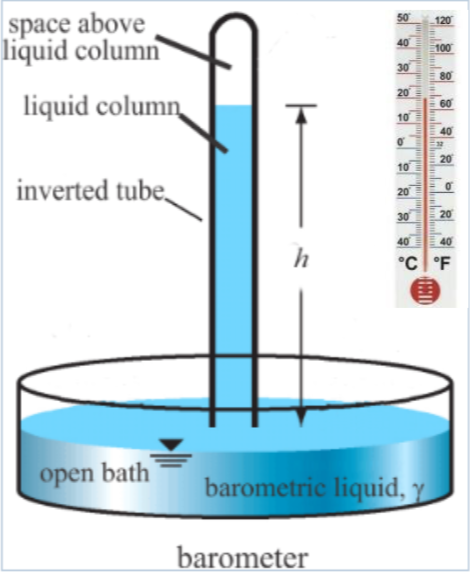
If the space above the mercury in the tube is a vacuum then nothing is. H m ftis. The mercury barometer is made of a glass tube sealed at the top.
If the atmospheric pressureat ground level is. Then the pressure at an altitude of. A simple mercury barometer used to measure atmospheric pressure The weight of the mercury in the tube is balanced by atmospheric pressure pushing down on the mercury in the tray If atmospheric pressure increases a greater length of mercury can be supported in the tube.
Atmospheric pressure pushes down on the mercury in the beaker which in turn pushes mercury up the tube. And the temperature is uniform at. The space at the top of the barometer tube is a vacuum and exerts no pressure on the mercury column.
The density is 13 600 kgm3. A manometer fig4 is used to measure the pressure of gas in a container. It contains mercury and the base of the tube dips into a beaker and below the surface of the mercury in the beaker.
Convert from inches of mercury read from the barometer to millibars. The barometric pressure equation shows this. Chamber for pressure 4 1150hPa old fhi dfashioned type not used todday 4Inspect temperature effects of an aneroid barometer in a chbhamber for temperature wihith a traveling standddard barometer.
Three additive corrections are used to calculate the local pressure from mercury barometer data. The unit mmHg is often called torr particularly in vacuum applications. The total pressure of the trapped air is now Hh cm of mercury where H is the atmospheric pressure in cm of mercury and h is in cm.
Here we can see that the space above the mercury column is filled with mercury vapors whose pressure is negligible so it can well be considered vacuum. If you know the inches of mercury simply multiply by 34433.

Barometer Atmospheric Pressure Mini Physics Learn Physics
Solved A Classic Mercury Barometer Has Only Mercury Vapour Chegg Com
What Is The Height Of A Mountain Where The Reading Of A Mercury Barometer Is 71cmhg At The Top Of The Mountain And 76cmhg At The Bottom Of The Mountain Where The
Post a Comment for "Mercury Barometer Formula"