Mercury Formula Of Oxide
MercuryII Oxide molecular weight. The chemical reaction that will yield both a liquid and a gas as products is 2HgO s 2Hg l O2 g.

Pin Von Wirabogroup Auf Formula
The SI base unit for amount of substance is the mole.

Mercury formula of oxide. Mainly used in rocket missile military prospecting areas. Mercury is a naturally occurring trace metalloid element and known neurotoxin with atomic symbol Hg atomic number 80 and atomic weight 20059. This is the formula for Copper II Oxide Copper has two possible oxidation numbers 2 or 1 since there are no written subscripts for in.
The empirical formula of dimethylmercury is C2H6Hg. Balancing Equations Ep06. It is a brownblack powder insoluble in water but soluble in nitric acid.
It is characterized as a heavy silvery-white metallic liquid at room temperature that is odorless. 1 grams MercuryII Oxide is equal to 00046170311197132 mole. The molecular formula for MercuryII Oxide is HgO.
The reactants space those substances v which we start here mercuryII oxide HgO is the reactant. It is a thermal decomposition reaction and the balanced equation can be written as. 2 HgOs 2 Hgl O2g we would know that the mercuryII oxide was a solid the mercury was a liquid and also the oxygen was a gas as soon as the reaction was carried out.
Average mass 216589 Da. It is a brownblack powder insoluble in water but soluble in nitric acid. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button.
Cherry Red metal liquid. What is the balanced chemical formula for mercury II oxide. The decomposition of mercury II oxide will produce liquid mercury as indicated by l and oxygen gas as indicated by g.
For example if the equation because that the decomposition the mercuryII oxide were written as. Mercury antimony oxide. 2 HgO 2 Hg O2.
Search by reactants HgO and by products Hg O 2. Using formulas us state this reaction as. Molar mass of HgO 2165894 gmol.
The chemical formula NOT. With hydrochloric acid it reacts to form calomel Hg 2 Cl 2. The formula for compound MercuryI oxide is Hg2O.
Mercuric II oxide yellow. Cherry Red liquid mercury. MercuryII oxide is a solid at room temperature and pressure.
Mercury II Oxide Mercury Oxygen. The balanced equation of mercuryII oxide HgO undergoes a chemical change to form mercury and oxygen is given as 2HgO s -- 2Hg l O2 g. Equation for mercury oxide is there are two possibillitiesMercuryI oxide mercurous oxide Hg2OMercuryII oxide mercuric oxide HgO.
Mercury II oxide is a solid at room temperature and pressure. Mercury II oxide also called mercuric oxide or simply mercury oxide has a formula of HgO. The balanced equation of mercuryII oxide HgO undergoes a chemical change to form mercury and oxygen is given as 2HgOs -- 2Hgl O2gThe reaction is a redox reaction.
In this video well write the correct formula for Mercury I oxide Hg2OTo write the formula for Mercury I oxide well use the Periodic Table and follow. Equations may be expressed in words. MercuryI oxide also known as mercurous oxide is an inorganic metal oxide with the chemical formula Hg 2 O.
The reactant side has four hydrogen atoms so the product side must also have four hydrogen atoms. The mineral form montroydite is very rarely found. Mercury antimony oxide Oxygen 8 atoms frame.
20059 159994 Percent composition by element. It has a red or orange color. Mercury I oxide also known as mercurous oxide is an inorganic metal oxide with the chemical formula Hg 2 O.
MercuryII oxide decomposes to mercury and oxygen. Mercury I oxide is toxic but without taste or smell. The exact same equation is repeated below with all the parts labeled.
Mercury II Oxide Mercury Oxygen. 2HgOsheat2HglO2g What is the exactly balanced equation of HgO Hg O2. Monoisotopic mass 217965515 Da.
Note that rounding errors may occur so always check the results. Convert grams MercuryII Oxide to moles or moles MercuryII Oxide to grams. Mercury reacts with oxygen to make mercuryII oxide.
MercuryII oxide also called mercuric oxide or simply mercury oxide has a formula of HgO. It has a red or orange color. Mercuric oxide HgO decomposes on heating to give mercury Hg and oxygen O2.
Mercury has been used in manufacturing as well as in dental and medical equipment fertilizers and pesticides. With hydrochloric acid it reacts to form calomel Hg 2 Cl 2.

Law Of Conservation Of Mass Google Search Chemical Equation Conservation Of Mass Equations
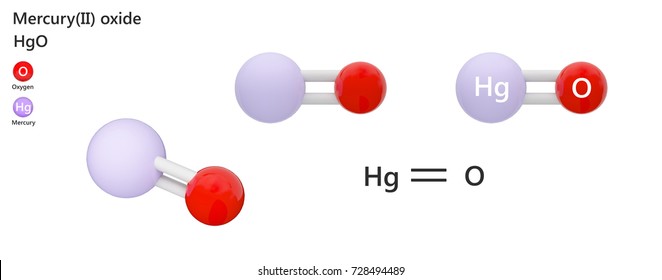
Mercury Ii Oxide Images Stock Photos Vectors Shutterstock
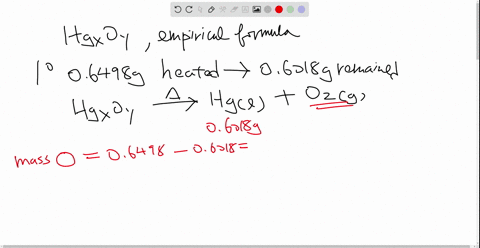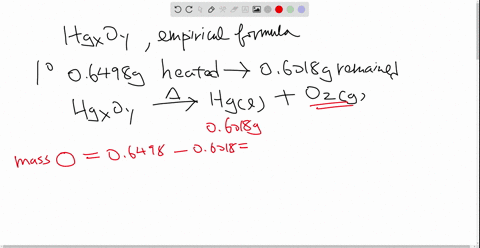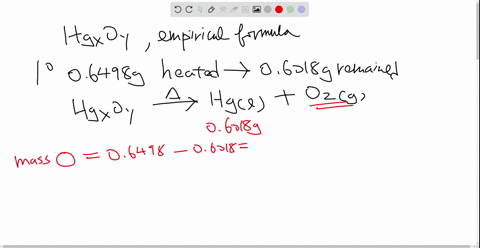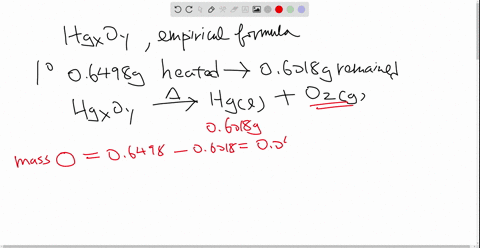
Solved Mercury Ii Oxide A Red Powder Can Be Decomposed By Heating To Produce Liquid Mercury And Oxygen Gas When A Sample Of This Compound Is Decomposed 3 87 Mathrm G Of Oxygen And 48 43 Mathrm G

Grade7 Icse Chemistry Languageofchemistry Objectiveswithanswers Languageofchemistryobjectiveswithanswers Chemistry E 7 Chemical
Post a Comment for "Mercury Formula Of Oxide"