Mercury-201 Protons Neutrons Electrons
Here we have 80 protons and 200 - 80 120 neutrons. Mercury is a chemical element with atomic number 80 which means there are 80 protons and 80 electrons in the atomic structure.

Mercury Protons Neutrons Electrons Electron Configuration
The chemical symbol for Mercury is Hg.
Mercury-201 protons neutrons electrons. How many electrons does Mercury have. See the example in the first row. The atomic symbol and the number of protons neutrons and electrons.
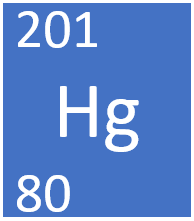
Mercury-201 is composed of 80 protons 121 neutrons and 80 electrons. Protons Neutrons and Electrons of all the Elements. Secondly what is the charge of mercury that has 80 electrons.
A neutral atom has the same number of protons as electrons so if you know the protons you also know the electrons. How many neutrons protons and electrons does mercury-201 have. So there must be 201 - 80 121 neutrons.
Proton Number Z 80. Use the same formula mass number minus atomic number to calculate the number of neutrons. Mercury-200 is composed of 80 protons 120 neutrons and 80 electrons.
Name Mercury Atomic Mass 20059 atomic mass units Number of Protons 80 Number of Neutrons 121 Number of Electrons 80. Helium has 2 protons 2 neutrons and 2 electrons. 121 neutrons 80 protons 80 electrons.
The mass number 201 is the total number of protons and neutrons. So there are 80 2 78 electrons. Besides how many neutrons are in Mercury 200.
Carbon has 6 protons 6 neutrons and 6 electrons. Hydrogen has 1 proton 0 neutron and 1 electron. Lithium has 3 protons 4 neutrons and 3 electrons.
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius. Also what has less protons than Mercury. The mass number 201 is the total number of protons and neutrons.
Melting Point-3887 C 23428 K -37966 F Boiling Point35658 C 62973 K 673844 F Number of ProtonsElectrons80. The atomic number is the number of protons in an atoms nucleus so we can tell right away that an atom of mercury contains 80 protons. Use the equation 202-80122 to find that mercury-202 has 122 neutrons.
A mercury atom needs 80 electrons to balance the 80 protons of protons 17 of neutrons 37 - 17 20 of electrons 17 - 0 17 of protons 16 the atomic number is not given but can be found on the periodic table of neutrons 32 - 16 16 of electrons 16 - -2 18. Since atoms are electrically neutral there must be as many electrons as there are protons. 1 Show answers Another question on Chemistry.
Neutron Number N 122. The 2 charge tells us that we have lost two electrons. How many neutrons does Mercury 201 have.
Melting Point K 23428. Mercury consists of two naturally occurring isotopes. Beryllium has 4 protons 5 neutrons and 4 electrons.
Nucleon Number A 202. How many neutrons and electrons does Mercury have. Mercury atoms have 80 electrons and 80 protons with 122 neutrons in the most abundant isotope.
Complete the information for each of the isotopes of mercury. Similarly how many isotopes are in Mercury. Isotope Protons Neutrons Electrons Isotople Symbol C carbon-14 6 8 6 mercury-200 mercury-201 I 2.
Atomic Number Z 80. Herein what is the number of neutrons in mercury. A mercury atom needs 80 electrons to balance the 80.
Mercury Mass Number Neutron Number Hg. What is the number of neutrons in mercury. This atom has protons neutrons and electrons.
Mercury has 80 electrons and protons and a variable number of neutrons - depending on the isotope. Also to know how many neutrons does Mercury 202 have. Usually gold is created from platinum which has one less proton than gold or from mercury which has one more proton than goldBombarding a platinum or mercury nucleus with neutrons can knock off an neutron or add on a neutron which through natural radioactive decay can.
G-factor g value 0. The atomic number is the number of protons in an atoms nucleus so we can tell right away that an atom of mercury contains 80 protons. Mass Number A 202.
Boron has 5 protons 6 neutrons and 5 electrons. In addition you may be interested in how many neutrons does Mercury 201 have. Boiling Point K 62988.
In the case of mercury the most common isotope is mercury-202. So there must be 201 - 80 121 neutrons. Click to see full answer.
An uncharged atom of mercury has an atomic number of 80 and an atomic mass of 200. In this video well use the Periodic table and a few simple rules to find the protons electrons and neutrons for the element Mercury Hg.

Mercury Element Key Stage Wiki

Mercury Atomic Structure Stock Image C045 6427 Science Photo Library

Mercury Protons Neutrons Electrons Electron Configuration
Chemical Elements Com Mercury Hg
Post a Comment for "Mercury-201 Protons Neutrons Electrons"