Mercury Atomic Number Definition
Members of a group typically have similar properties and electron configurations in their outer shell. A horizontal row in the periodic table.
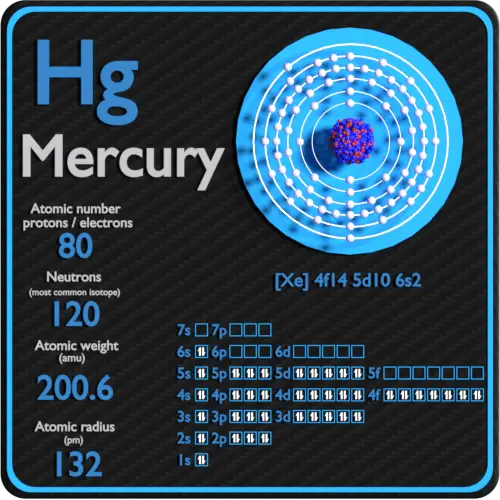
Mercury Protons Neutrons Electrons Electron Configuration
The number of atoms in 1 mole of carbon is.

Mercury atomic number definition. While the atomic number always stays the same some elements have atoms with different atomic mass numbers. Mercury the metal is highly toxic and its used very little anymore as a result. Atomscm 3 of a pure material having atomic or molecular weight M.
An electrically neutral atom of mercury atomic number 80 has. The commercial unit for handling mercury is the flask which weighs 76 lb. Total number of protons in the nucleus is called the.
Mercury A liquid metallic element atomic number 80. Spain and Italy produce about 50 of the worlds supply of the metal. Gramsmol and the material density.
The combined number of protons and neutrons in an atom is called the atomic mass number. 80 protons and 80 electrons. The number of protons in the nucleus is called the atomic number.
2-8-18-32-18-2 or Xe4f 14 5d 10 6s 2. Which is symbolized by the symbol Hg and its atomic number 80 and it is called by other names among them the black cinnabar and it is found in nature as a raw element knowing that mercury is one of the toxic elements that cause many damages to the human body if it enters it including the destruction of the nervous system and the kidney. Gramcm 3 is easily computed from the following equation using Avogadros number N A 602210 23 atoms or molecules per.
Atoms by definition are electrically neutral. Atomic weight 20059 often obtained from cinnabar a major mercury ore. - Damage to brain functions.
It only rarely occurs free in nature. Found in Egyptian tombs of 1500 BC. Mercury has been used in manufacturing as well as in dental and medical equipment fertilizers and pesticides.
Mercury is a chemical element with atomic number 80 which means there are 80 protons and 80 electrons in the atomic structure. 35662 C 67391 F specific gravity. 3883 C 3789 F boiling point.
Mercury has a number of effects on humans that can all of them be simplified into the following main effects. Thus the atomic number of Na atom number of electrons number of protons 11. Mercury is commonly known as quicksilver and was formerly named hydrargyrum.
Atomic number Number of protons. The definition of mercury is a heavy silver-white metal with an atomic number of 80 that is liquid at ordinary temperatures. The chemical symbol for Mercury is Hg.
The atomic number density N. The atomic number density N. 135 at 20 C 68 F valence.
- Disruption of the nervous system. Mercury is a chemical element with atomic number 80 which means there are 80 protons in its nucleus. For example cadmium generally is considered a heavy metal with an atomic number of 48 and specific gravity of 865 while gold typically is not toxic even though it has an atomic number.
This is because some elements have a different number of neutrons in the nucleus. Protons carry a positive charge so the nucleus of an atom of mercury carries a charge of 80. Some light metals or metalloids are toxic while some high-density metals are not.
- DNA damage and chromosomal damage. Mercurys atomic number is 80 and was discovered somewhere around 1500 BC. The atomic number is the number of protons in an atoms nucleus so we can tell right away that an atom of mercury contains 80 protons.
Mercury is the only common metal liquid at ordinary temperatures. Atomscm 3 is the number of atoms of a given type per unit volume V. The atomic number of each element is unique.
The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. For example in a sodium atom there are 11 electrons and 11 protons. Cm 3 of the material.
A vertical column in the periodic table. - Allergic reactions resulting in skin rashes tiredness and headaches. Mercury is a naturally occurring trace metalloid element and known neurotoxin with atomic symbol Hg atomic number 80 and atomic weight 20059.
Avogadros constant is definition the number of particles in 1 mole of a pure substance. Known to ancient Chinese and Hindus. Its named after the planet Mercury.
It is characterized as a heavy silvery-white metallic liquid at room temperature that is odorless. The mass of 1 mole of chromium atomic. The chief ore is cinnabar.

Mercury Element Information Properties And Uses Periodic Table

Mercury Protons Neutrons Electrons Electron Configuration

Element Definition Facts Properties And Uses

Mercury Element Facts Hg Or Atomic Number 80 Atomic Number Electron Configuration Ionization Energy
Post a Comment for "Mercury Atomic Number Definition"